

One of the chief responsibilities of a nuclear medicine technologist is safety. The images obtained by the technologist are interpreted by a specially trained physician.
#Nuclear fission uranium 235 equation explained series
The apparatus may be as simple as a piece of photographic film or as complex as a series of computer-controlled electronic detectors. Nuclear medicine technologists administer the substances containing the radioactive isotope and subsequently operate the apparatus that detects the radiation produced by radioactive decay. A nuclear medicine technologist has similar responsibilities, using compounds containing radioactive isotopes to help diagnose and treat disease. Generally speaking, a radiological technician deals with X ray equipment and procedures.
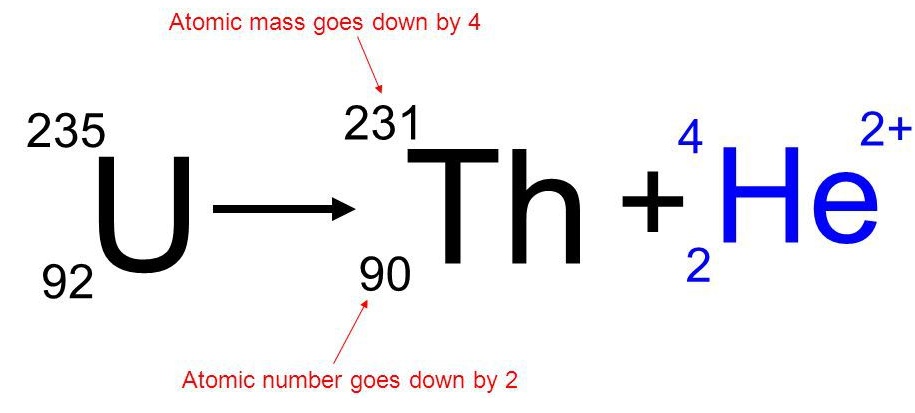
Currently, researchers are looking for safe, controlled ways of producing useful energy using fusion.Ĭareer Focus: Nuclear Medicine Technologist The conditions necessary for fusion can be created using an atomic bomb, but the resulting fusion is uncontrollable (and the basis for another type of bomb, a hydrogen bomb). Currently, the only known stable systems undergoing fusion are the interiors of stars. The practical problem is that to perform fusion, extremely high pressures and temperatures are necessary. In addition, the product of fission is helium gas, not a wide range of isotopes (some of which are also radioactive) produced by fission. On a mass (per gram) basis, however, the hydrogen fusion emits many times more energy than fission does. Notice that the amount of energy given off per mole of reactant is only a fraction of the amount given off by the fission of 1 mol of uranium-235. While the steps of the process are complicated, the net reaction is: One example is the hydrogen fusion, which makes helium. In this process, smaller nuclei are combined to make larger nuclei, with an accompanying release of energy.
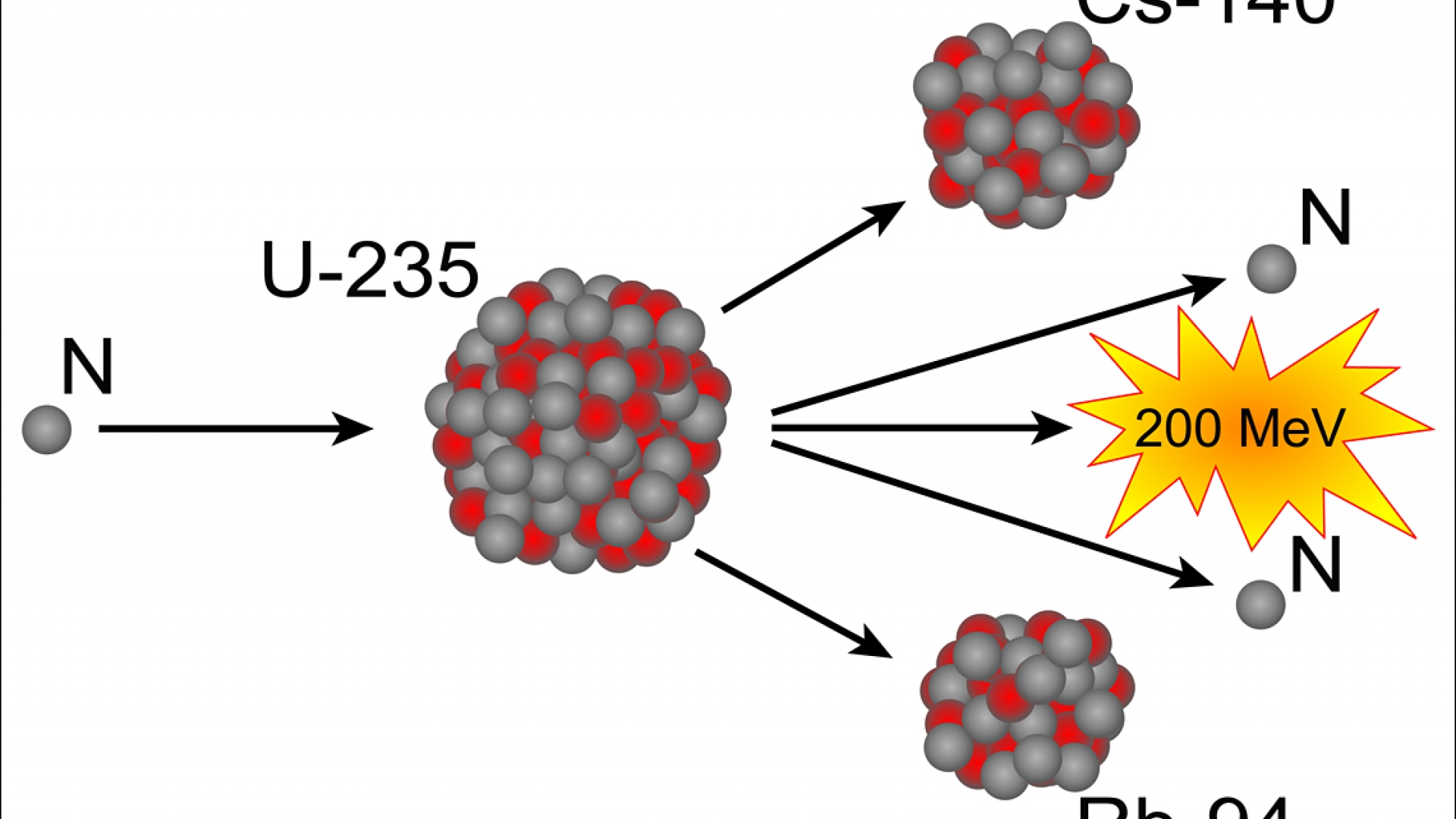
At lesser concentrations, the chain reaction cannot sustain itself, so no explosion is produced.įusion is another nuclear process that can be used to produce energy. To be useful in an atomic bomb, the uranium in uranium-235 must be enriched to 70% or more. Enrichment of uranium is a laborious and costly series of physical and chemical separations. (Remember that the radioactive process that a nucleus undergoes is characteristic of the isotope.) To make uranium useful for nuclear reactors, the uranium in uranium-235 must be enriched to about 3%. The first controlled chain reaction was achieved on December 2, 1942, in an experiment supervised by Enrico Fermi in a laboratory underneath the football stadium at the University of Chicago.Īlthough fairly simple in theory, an atomic bomb is difficult to produce, in part because uranium-235, the isotope that undergoes fission, makes up only 0.7% of natural uranium the rest is mostly uranium-238, which does not undergo fission. We predict that the overall process will give off five neutrons. Consider the following nuclear reaction, in which the molar mass of each species is indicated to four decimal places: Where does this energy come from? If we could precisely measure the masses of the reactants and the products of a nuclear reaction, we would notice that the amount of mass drops slightly in the conversion from reactants to products. Nuclear changes occur with a simultaneous release of energy. Describe the difference between fission and fusion.Explain where nuclear energy comes from.


 0 kommentar(er)
0 kommentar(er)
